Structural composition of CAR-T vector plasmid: a comprehensive overview
Chimeric antigen receptor (CAR) T cell therapy revolutionizes cancer treatment by leveraging the special targeting of the immune system and the inherent ability to destroy malignant cells. This personalized treatment involves genetically designing the patient’s own T cells to express tumor-specific antigens (MHCs) independent of the major histocompatibility complex (MHC). At the heart of this technology is the CAR-T vector plasmid: a synthetic DNA construct encoding the CAR protein and contains all the necessary regulatory elements to ensure proper expression and function in T cells.
Understanding the structural components of CAR-T vector plasmids is not only essential for optimizing therapeutic efficacy and safety, but is also essential for promoting the field of cellular immunotherapy. This article discusses in detail the basic vector elements contained in the automotive structure itself and the plasmids for effective automotive expression, selection and function.
1. Automotive protein: modular structure and function
At the heart of CAR-T therapy is chimeric antigen receptors, a synthetic transmembrane protein that makes T cells comparable to the ability to recognize antigens associated with specific tumors and initiate a cytotoxic immune response. Cars are modular and usually consist of three key areas:
-
Exogenous domain (extracellular antigen recognition)
-
Transmembrane domain (membrane anchoring)
-
Intraditional domain (intracellular signaling and activation)
Each domain is carefully designed to maximize T cell specificity, activation and durability.
1.1 Exogenous Domain: Accurate Targeting of Tumor Antigens
The extracellular domain protrudes from the T cell surface and mediates antigen recognition. It contains three important subcomponents:
a) Signal peptide
Signal peptides are usually derived from the CD8α leadership sequence, a short amino acid sequence at the N-terminal, directing the nascent CAR protein to the endoplasmic reticulum. This promotes proper folding, glycosylation and transport to the cell surface. Without effective signal peptides, automotive proteins may not reach the membrane, making the treatment ineffective.
b) Antigen binding domain (SCFV)
This domain confers target specificity. It is usually a single-chain variable fragment (SCFV), which is a fusion of variable regions of the monoclonal antibody weight (VH) and light (VL) chains linked by a flexible peptide. SCFV binds directly to tumor antigens on the cell surface and is independent of MHC performance, allowing CAR-T cells to bypass the tumor immune evasion mechanism that downregulates MHC molecules.
The affinity and specificity of SCFV seriously affect the treatment outcome. While high affinity can improve target recognition, overly powerful binding may lead to activation-induced cell death or off-target toxicity. In addition, epitope positions on antigen and antigen density can also affect CAR-T efficacy. It is worth noting that the SCFV domains are prone to aggregate, which may lead to nutrition (antigen independent) signaling, resulting in T cell depletion. Therefore, engineering stable, well-folded SCFV is a continuous field of research.
c) hinge or gasket area
The hinge region connects SCFV to the transmembrane domain and provides structural flexibility, allowing the antigen-binding domain to reach and participate in target epitopes that may be spatially hindered. The length and composition of the gasket significantly affect the function of the car.
Commonly used gaskets include hinges from CD8α or CD28 or IgG FC regions (eg IgG1 or IgG4). IgG-derived isolation agents are often necessary to prevent interaction with FC gamma receptors (FCγR) on innate immune cells, which may trigger unexpected immune responses and CAR-T cell clearance. The gasket length must be tailored to specific antigenic epitopes; longer spacers help access to membrane-transparent epitopes, but may increase nourishing signaling and activation-induced cell death, while shorter spacers are more suitable for membrane-biased epitopes.
In addition to functional effects, the gasket can also act as epitope for antibody-based automotive detection or purification techniques, such as merging of Streptococcus-TAG sequences.
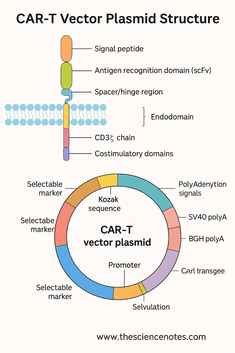
1.2 Transmembrane domain: anchoring and stability
The transmembrane domain anchors the vehicle in the T cell membrane and physically connects the extracellular recognition domain to the intracellular signaling module. Despite its small size, this domain affects automotive expression levels, receptor stability, and signaling.
The transmembrane domains usually come from native T-cell proteins, such as:
-
CD3ζ (CD247)
-
CD4
-
CD8α
-
CD28
The choice of transmembrane domains can affect the integration of automotive with endogenous T cell receptor (TCR) complexes. For example, the CD3ζ transmembrane domain can promote CAR incorporation into native TCR complexes, which may enhance activation, but may destroy stable receptor complexes. The CD8α and CD28 transmembrane domains tend to confer greater receptor stability and consistent surface expression. In addition, transmembrane domains may affect cytokine release profiles and nutrition signaling, which are closely related to safety and efficacy.
1.3 Intracellular signaling domain: initiates T cell activation
The intracellular domain converts antigen recognition into T cell activation and cytotoxicity responses. It contains signaling motifs that trigger proliferation, cytokine release, and target cell killing.
A) CD3ζ chain
The CD3ζ chain is the main signaling domain in all automotive designs. It contains three immune receptors tyrosine based on activation motifs (ITAMS), which when phosphorylated on antigen binding initiates a downstream signaling cascade necessary for T cell activation.
b) Co-stimulation domain
To enhance T cell function, durability and prevent exhaustion, CAR is incorporated into one or more costimulatory domains upstream of CD3ζ:
-
CD28: Promotes rapid expansion of T cells and efficient cytokine secretion, but may lead to shorter durability.
-
4-1BB (CD137): Enhance T cell survival and memory formation, leading to continuous activity.
-
Others: ICOS, CD27, OX40 and combinations (such as MyD88 Plus CD40) are under investigation for optimized signaling.
CAR generations are classified based on the complexity of their intracellular domains:
-
First generation car: CD3ζ only.
-
Second generation car: CD3ζ plus a costimulus domain (most FDA-approved cars belong here).
-
Third-generation car: CD3ζ plus two costimulation domains to potentially enhance function.
2. Automobile-T vector plasmid: an essential genetic element
CAR transgenes are embedded in plasmid vectors to maximize expression, stability, and selection ability during T cells and plasmid transmission.
2.1 Promoter: Driving a car expression
The effective transcription of automotive genes depends on the selection of promoters that control expression cassettes. Common promoters include:
-
EF1α (elongation factor 1α): Powerful configuration promoters are active in a wide range of cell types, including T cells. It can achieve stable high-level expression without silence.
-
CMV (cytomegalovirus) immediate promoter: Viral promoters with robust early expression, but can be silenced in some primary cells over time.
-
PGK (phosphoglycerate kinase) promoter: When moderate expression is required, weaker configuration promoters are used to reduce stress signaling or toxicity.
Promoter selection can balance the need for sufficient CAR proteins on the surface and minimize the risk of overactivation or exhaustion.
2.2 Kozak sequence: Optimized translation startup
The Kozak consensus sequence (GCCGCCACC) immediately upstream of the initiation codon (AUG), enhancing ribosome binding and initiating efficient translation of CAR mRNA. Combining optimized Kozak sequences is a standard practice for maximizing protein yield.
2.3 Polyadenylation signal: Ensure mRNA stability
Following the CAR coding sequence, polyadenylation (PolyA) signaling promotes appropriate transcription termination, mRNA stability, and nuclear export. The two commonly used polya sequences are:
-
SV40 late Polya: From alternative virus 40, widely used in mammalian expression vectors.
-
BGH Polya: The polyadenylation sequence of bovine growth hormone is also effective in stabilizing the transcript.
Correct polyadenylation reduces degradation and enhances CAR protein production.
2.4 Optional markers and reporter genes
To identify and select successfully transfected or transduced T cells, plasmids usually include:
-
Antibiotic resistance genes (e.g., neomycin resistance): Antibiotic selection is allowed during cell culture.
-
Fluorescent proteins (e.g. EGFP, MCHERRY): Enable visualization and classification via flow cytometry.
-
Double mark: Combined antibiotic resistance and fluorescence for flexible selection.
These elements are usually controlled by separate promoters and do not interfere with automotive expression.
2.5 Origin of Copy (ORI)
For plasmid spread in bacteria, the origin of replication, e.g. puc ori include. This allows E. colipromote large-scale plasmid production required for clinical and research applications.
2.6 Bacterial selection markers
this Ampicillin resistance gene (AMP^r) Antibiotic selection in bacterial cultures ensures that only the bacteria that make up the plasmids.
3. FDA-approved CAR-T Products: Real-world Vector Examples
Several CAR-T cell therapies have been approved by the FDA, each using a different but fundamentally similar vector design.
| Business name | Common Name | company | Approval date | Target antigen | Indications |
|---|---|---|---|---|---|
| Kymriah™ | tisagenlecleucel | Nova | August 2017 | CD19 | Relapsed/refractory B-ALL |
| Yescarta™ | axibabtagene cileoleucel | Gilead/Kite | October 2017 | CD19 | Relapsed/refractory DLBCL |
| tecartus™ | Brexucabtagene Autoleucel | Gilead/Kite | July 2020 | CD19 | Relapsed/refractory mantle cell lymphoma |
| Breyanzi® | Lisocabtagene Maraleucel | Bristol Myers Squibb | February 2021 | CD19 | Relapsed/refractory large B-cell lymphoma |
| Abecma® | idecabtagene speed | Bristol Myers Squibb | March 2021 | BCMA | Relapsed/refractory multiple myeloma |
| CARVYKTI® | Ciltacabtagene automatic race | Janson | February 2022 | BCMA | Relapsed/refractory multiple myeloma |
These products embody how modular automotive and vector designs adapt to targeting different antigens and diseases while maintaining the core plasmid backbone structure.
https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
4. Conclusion: The Future of Car-T Vector Design
CAR-T vector plasmids are far more than simple DNA transport vehicles. This is an essential complex, modulated genetic tool for successful CAR-T cell therapy. Each component (from the affinity and interval length of SCFV from the selection of transmembrane and signal domains to promoter strength and selection markers) affects treatment outcomes.
Despite significant clinical success in first- and second-generation cars, ongoing challenges include antigen escape, T cell depletion, and toxicity. These questions highlight the need for vector design, including inducible promoters, novel costimulatory domains, optimized SCFVs and safety switches.
As CAR-T therapy extends beyond hematologic malignancy to solid tumors and other diseases, vector plasmids will need to evolve through advanced regulatory elements and carefully regulate automotive architectures to address new challenges. Enhanced high-throughput screening and computing design tools will accelerate this development, ultimately improving efficacy, safety and accessibility for patients around the world.


