Determination of carbohydrates, starches, proteins and lipids in different solutions
introduce
Carbohydrates, proteins, lipids, and nucleic acids are the basic organic molecules found in living organisms. These biological macromolecules contain carbon and may also contain hydrogen, oxygen, nitrogen, phosphorus, sulfur and other trace elements. Carbohydrates are composed of monosaccharide units and mainly play the role of energy storage (Mika et al., 2024). Various methods are used to determine the presence of macromolecules. For example, the presence of reducing sugars is detected using Benedict’s reagent, which changes color from green to reddish upon oxidation of copper ions (Benedict, 2002). Starch is a polysaccharide with long chains of glucose units and can be identified by the iodine test, which turns blue-black in the presence of starch due to the interaction of iodine with the helical structure of the polymer. Proteins are composed of long-chain amino acids and play key roles in enzymatic activity, molecular transport, and structural support (Pesek et al., 2022). The biuret test detects proteins by forming purple complexes with copper ions bound to peptide bonds (Lubran, 1978). Lipids include fats, oils, and phospholipids, which are primarily involved in energy storage and membrane structure. Confirm the presence of lipids using the oil spot test, in which the lipids leave a translucent mark on the paper (oil spot test, n.d.).
In this experiment, Benedict’s test, iodine test, biuret test and oil spot test were performed to qualitatively determine the presence of carbohydrates, starch, proteins and lipids in various solutions, respectively (Mika et al., 2024 ). The hypothesis of this experiment is that if carbohydrates, starches, proteins, and lipids are present in the test solution, the Benedict test will produce a color change indicating the presence of reducing sugars, while the iodine test will result in a blue-black color development confirming starch, double The uret test will produce a purple color, indicating the presence of proteins, while the oil spot test will show translucent markings, indicating the presence of lipids.
Materials and methods
To detect carbohydrates, Benedict’s test was performed (Mika et al., 2024). Prepare eight test tubes, each containing 10 drops of a different solution: onion juice, potato juice, sucrose solution, glucose solution, distilled water, fructose solution, starch solution, and chyme. Add 1 ml of Benedict’s reagent to each test tube and record the initial color. Then place the test tube in a boiling water bath for 3 minutes. After cooling, the final color of each solution was recorded and analyzed to determine the presence of carbohydrates.
For starch testing, the iodine test follows a similar procedure to the Benedict test. Instead of Benedict’s reagent, add 4-6 drops of iodine solution to each test tube containing the same solution set (Mika et al., 2024). No boiling was performed in this test.
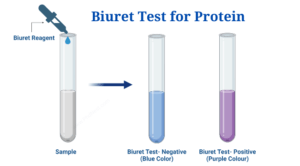
Assess the presence of protein using the biuret test. Prepare six test tubes and add 2 ml of each of the following solutions: egg white, honey, amino acids, distilled water, protein solution and chyme. Record the initial color of each solution. Add 1 ml of 2.5% NaOH solution to each test tube, followed by 8-10 drops of biuret reagent. Gently mix the solution and observe any color changes to determine the presence of protein (Mika et al., 2024).
Detect lipids using an oil spot test. Cut six small squares from the brown paper bag and label them accordingly. Place a drop of each of the following on individual cubes: maple syrup, chocolate syrup, canola oil, peanut butter, icing sugar, and chyme. Allow the squares to dry and observe the presence of translucent grease spots to confirm the presence of lipids (Mika et al., 2024).
result
In Benedict’s test, only the onion juice changed color from blue to green, indicating the presence of reducing sugars. All other samples remained blue, indicating the absence of reducing sugars.
Table 1: Benedict test results
| tube | solution (10 drops) | initial color | Color after cooking |
| 1 | onion juice | white | green |
| 2 | potato juice | brown | blue |
| 3 | sucrose solution | Clear | blue |
| 4 | glucose solution | Clear | blue |
| 5 | distilled water | Clear | blue |
| 6 | fructose solution | Clear | blue |
| 7 | starch solution | Clear | blue |
| 8 | chyme | Creamy yellow | blue |
In the iodine test, all solutions turn brown after adding iodine, indicating that there is no starch in any sample.
Table 2: Iodine test results
| tube | solution (10 drops) | initial color | color after iodine |
| 1 | onion juice | white | brown |
| 2 | potato juice | brown | brown |
| 3 | sucrose solution | Clear | brown |
| 4 | glucose solution | Clear | brown |
| 5 | distilled water | Clear | brown |
| 6 | fructose solution | Clear | brown |
| 7 | starch solution | Clear | brown |
| 8 | chyme | Creamy yellow | brown |
The biuret test shows the presence of protein in ovalbumin, protein solutions, and chyme, as evidenced by a purple change. All other samples remain blue, indicating the absence of protein
Table 3: Biuret test results
| tube | Solution (2 ml) | initial color | Color after biuret |
| 1 | Ovalbumin solution | Clear | purple |
| 2 | honey solution | yellow | blue |
| 3 | Amino acid solution | yellow | blue |
| 4 | distilled water | Clear | blue |
| 5 | protein solution | Clear | purple |
| 6 | chyme | light yellow | purple |
In the oil spot test, lipids are detected in canola oil, peanut butter, and frosting, which show up as large, visible oil spots. No oil spots were observed in other samples.
Table 4: Oil spot test results
| tube | solution (1 drop) | Description of oil spot reaction |
| 1 | maple syrup | The spots are not moving and there is no grease |
| 2 | chocolate syrup | The spots are not moving and there is no grease |
| 3 | rapeseed oil | The spots become larger and oily |
| 4 | peanut butter | The spots become larger and oily |
| 5 | frosting | The spots become larger and oily |
| 6 | chyme | Spots have no movement, liquid, no grease |
discuss
The results support the hypothesis that the presence of carbohydrates, starches, proteins and lipids leads to specific color changes – the appearance of green, orange, brick red, purple and oily spots – when the respective reagents are added. Qualitative analysis of samples evaluated for color change can help identify specific macromolecules in solution.
Benedict’s test showed the presence of reducing sugars in the onion juice and the color of the onion juice changed to green (Table 1). This color change confirms the presence of reducing sugars, as they can reduce copper ions to cuprous oxide. In contrast, other samples did not change color, indicating the absence of reducing sugars (Benedict, 2002).
Iodine testing showed no presence of starch in any sample, including the starch solution itself (Table 2). Typically, iodine forms a blue-black complex with starch, but the lack of color change in our tests suggests that the starch is degraded, or that the iodine solution is ineffective (Pesek et al., 2022). Starch solutions have a limited shelf life and may degrade over time, which could explain negative results. Additionally, problems with the iodine solution may also contribute to lack of response.
The biuret test showed the presence of protein in ovalbumin, protein solutions and chyme, as evidenced by the purple change (Table 3). The lack of color change in amino acid solutions can be explained by the lack of peptide bonds; biuret reagents react exclusively with peptide bonds, whereas single amino acids do not contain these bonds and therefore do not undergo color change (Lubran, 1978).
In the oil spot test, lipids were detected in canola oil, peanut butter, and icing sugar, producing visible oil spots (Table 4). The absence of greasy spots in maple syrup, chocolate syrup, and chyme confirmed the absence of lipids in these samples. Oil spot tests are effective for detecting lipids because of their ability to produce an oily residue on paper (oil spot test, n.d.).
To increase experimental reliability, it is important to use freshly prepared solutions and include positive and negative controls in each test. For example, the iodine test for starch lacks a positive control, making it difficult to accurately interpret the results. Including known positive samples can clarify whether the problem is the starch solution or the iodine reagent.
Further experiments
To enhance the analysis of carbohydrates, starches, proteins, and lipids, quantitative methods such as colorimetric assays and Soxhlet extraction can be used to more accurately measure concentrations. Furthermore, the combination of specific enzyme activity assays and advanced techniques such as thin layer chromatography will help to understand the biochemical properties and types of these macromolecules.
refer to
- Benedict SR (2002). Reagents for detecting reducing sugars. 1908. journal of biochemistry, Chapter 277(16), e5.
- Rubran MM (1978). The total serum protein was determined using the biuret method. Annals of Clinical and Laboratory Sciences, 8(2), 106-110.
- Grease spot test. (nd). Lipid identification method. Taken from [ s.pdf]
- Pesek, S., Lehene, M., Brânzanic, AMV, & Silaghi-Dumitrescu, R. (2022). On the origin of blue color in iodine/iodide/starch supramolecular complexes. Molecules (Basel, Switzerland), 27(24), 8974.
https://doi.org/10.3390/molecules27248974
- Mika (TA), Klein (RJ), Brejan (AE), Connaugh (RL), Swimmer (LM), White (R).
In E., Gosses, MW, Carter, TE, Andrews, AM, Maier, JL, & Sidiq, F. (Eds.). (2024). Anatomy and Physiology BIO 211 Laboratory Manual (3rd ed.). Owens Community College.
Amino Acids: Types, Functions, Sources, and Differences between Essential and Non-Essential Amino Acids (thesciencenotes.com)

